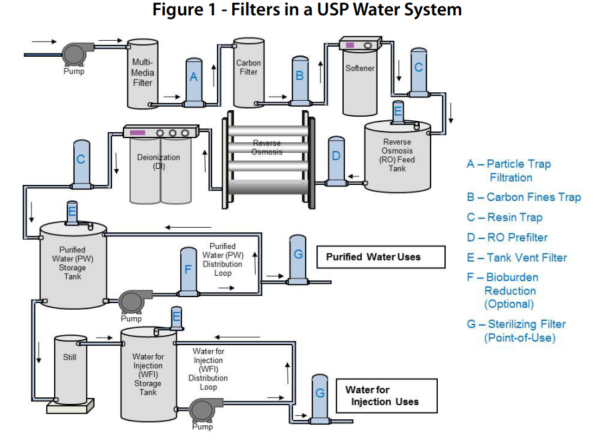
Usp bacteriostatic water for injection usp sterile water for irrigation the usp designation means that the water is the subject of an official monograph in the current us pharmacopeia with various.
Water for injection usp conductivity.
Know the specification of water for injection wfi as per united states.
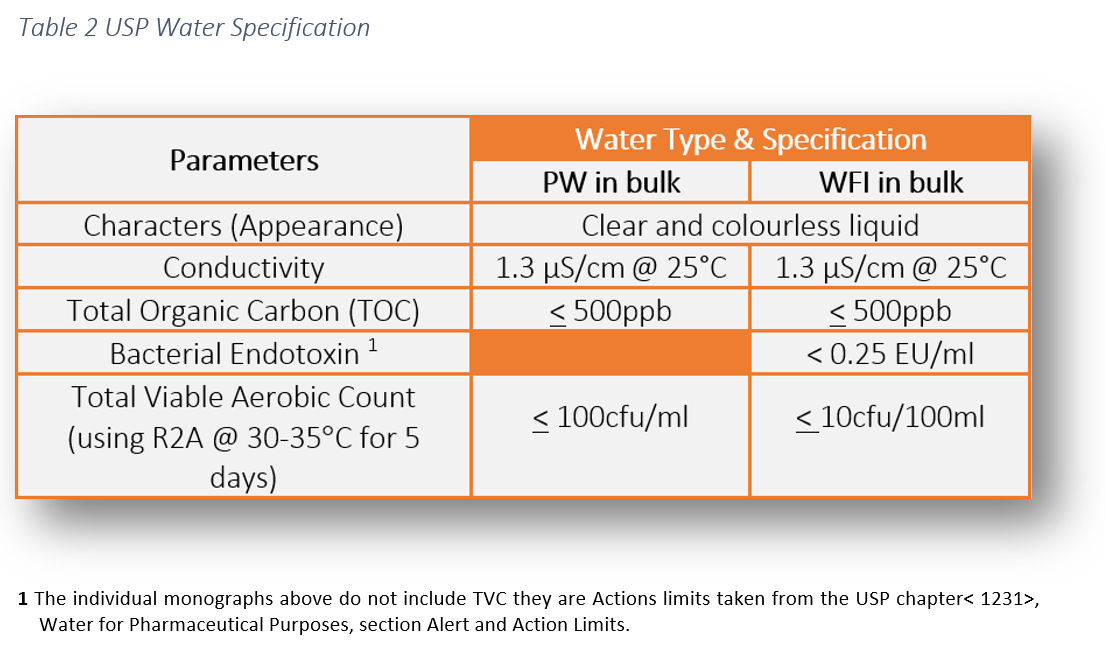
Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use.
Conductivity at 25 c less than 1 3 ms cm.
Water conductivity is also affected by the presence of.
Injectable drugs whose solvent is water.
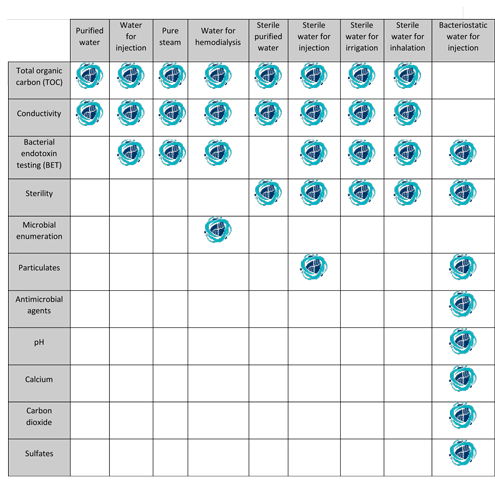
The procedure described in the section bulk water is designed for measuring the conductivity of waters such as purified water water for injection water for hemodialysis and the condensate of pure steam produced in bulk.
Know the specification of water for injection wfi as per united states pharmacopoeia.
1752 1754 and the general chapters 643 toc 645 water conductivity p.
Some gases notably co 2 readily dissolve in water and interact to form ions which predictably affect conductivity.
The usp united states pharmacopeia defines this as highly purified waters containing less than 10 cfu 100 ml of aerobic bacteria.
1927 1929 and 1231 water for pharmaceutical purposes p.
Usp24 contains complete versions of all pharmaceutical water monographs p.
Water for injection by definition is water that is intended for use in the manufacture of parenteral i e.
For water packaged in bulk but manufactured elsewhere or for sterile purified water sterile water for injection sterile.
Replacing the heavy metals attribute was considered unnecessary because a the source water specifications found in the npdwr for individual heavy metals were tighter than the approximate limit of detection of the heavy metals test for usp xxii water for injection and purified water approximately 0 1 ppm b contemporary water system.
Ankur choudhary print question.
These waters should also have fewer than 500 ppb of total organic.
Clear colorless and odorless liquid.
The conductivity of the ubiquitous chloride ion at the theoretical endpoint concentration of 0 47 ppm when it was a required attribute test in usp xxii and earlier revisions and the ammonium ion at the limit of 0 3 ppm represents a major portion of the allowed water impurity level.
Water for injection packaged in bulk for commercial use elsewhere meets the requirement of the test for bacterial endotoxins as indicated below and the requirements of all the tests under sterile purified water.
You may purchase usp24 by calling customer service at 800 877 6733.
A balancing quantity of cations such as sodium ion is.
The usp is also available at pharmacy colleges.
We can not provide photocopies of copyrighted material.
The tests for total organic carbon and water conductivity apply to water for injection produced on site for use in manufacturing.